PRODUCT DESCRIPTION
Donor
Deceased Australian donor.
Source Tissue
Human femur.
Treatment
Antibiotic - gentamicin.
Process Method
Femoral head and soft tissue removed. Allografts vary in length.
QC Testing
Microbiological culture - sampled throughout retrieval and processing including just prior to final packaging.
Radiological review of all source bone.
Packaging
Individually triple packed.
Irradiation
Product is able to be supplied non irradiated or irradiated.
Where irradiated (indicated by a red sticker) the product has been irradiated to a minimum dose of 25 kGy within its final packaging.
CLINICAL APPLICATIONS
Restoration/repair of musculoskeletal tissue.
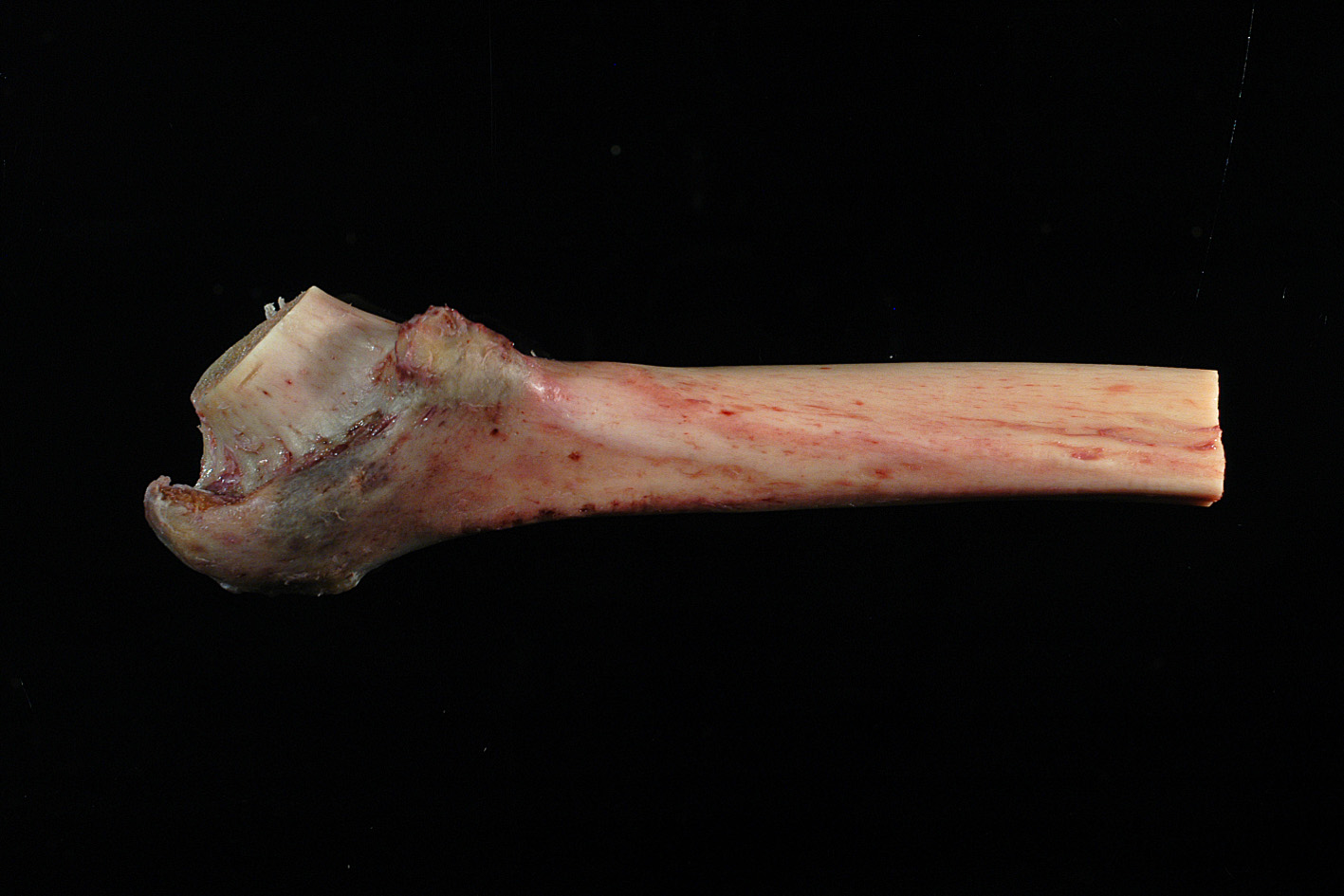
Femur – Proximal (Shaft, Trochanter and Neck)
STORAGE
Below -40°C until expiry date or between -20°C and -40°C for up to 6 months or expiry date (whichever is sooner) in a continually monitored and alarmed freezer.
TRANSPORT
Transported on dry ice to maintain temperature below -40°C.
ORDERING INSTRUCTIONS
Where possible place order 2 business days prior to the delivery date.
Where non irradiated product is ordered the surgeon will need to sign a disclaimer acknowledging that the product is non irradiated.
| Product | Billing Code |
|---|---|
| Femur - Proximal (Shaft, Trochanter and Neck) | TBV25 |
